Testing continues to be a critical component in limiting the spread of COVID-19. Solutions that increase workflow efficiency can help diagnostic laboratories improve productivity by increasing sample processing capacity and accuracy.
A COVID-19 test center in Japan provides saliva-based qPCR testing. Here the team describes their test method and how they have incorporated Gilson liquid handling tools into their workflow to help improve efficiency and increase testing throughput.
Customer Profile: Dr. Yukumasa Kazuyama, PhD, SB Coronavirus Inspection Center Corporation
Dr. Yukumasa Kazuyama, PhD, is the director of the SB Coronavirus Inspection Center Corporation. He is a former member of the Japanese Society for Virology and the Japanese Society of Clinical Virology, and former manager and CEO of KITASATO-OTSUKA Biomedical Assay Laboratories Co., Ltd. He has published more than 70 papers in Japanese and English.
Saliva-based qPCR Testing
SB Coronavirus Inspection Center Corporation, a subsidiary of SoftBank Group Corporation in Japan, was established to prevent the spread of COVID-19 and normalize Japanese economic activities as quickly as possible. The center provides an affordable saliva-based testing service and performs COVID-19 tests for private companies, universities, sports associations, local governments, and individual customers.
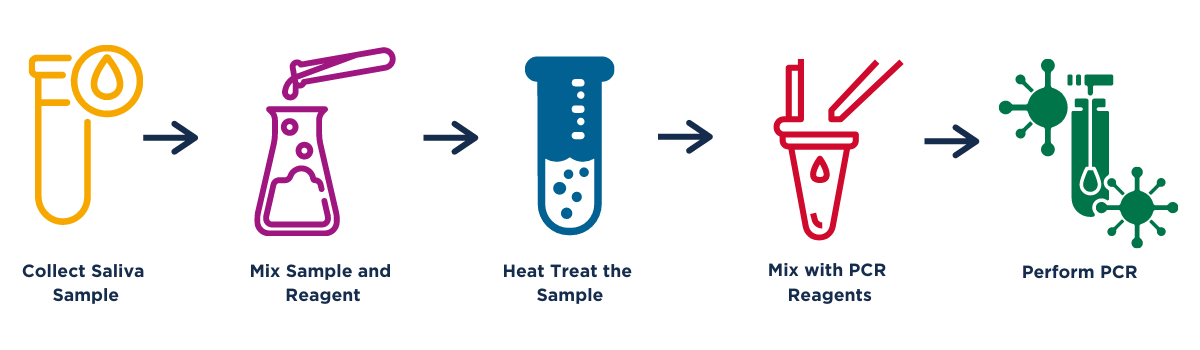
The center uses a virus-deactivated saliva qPCR test (Figure 1). According to Dr. Yukumasa Kazuyama, director of the center, the benefits of using a saliva-based test include more comfort for the examinee and less risk of infection to medical personnel. Kazuyama says that although there have been concerns that saliva-based testing is not as sensitive as tests using nasal swabs, they have established conditions for sample collection, including no smoking, eating, or drinking except water, and no toothbrushing within one hour before the test, to enable reliable testing. In addition, he says that results from nasal swab tests and saliva-based tests have a high rate of agreement, as determined by a research institute under the supervision of a Japanese governmental office.
Figure 1. Schematic of qPCR testing process. Samples are tested individually. A Ct value < 40 indicates the virus was detected (probable positive). Results are typically obtained within 1-2 days.
Tools to Help Increase Testing Throughput
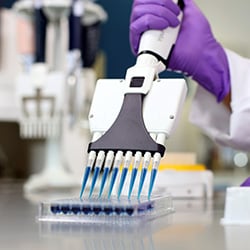
The center has used a variety of PIPETMAN® pipettes and PLATEMASTER® for dispensing steps in their protocol. However, after integrating PIPETMAX® into their workflow, these steps became even more efficient.
PIPETMAX
PIPETMAX is a compact, benchtop-sized automated pipetting solution for efficient processing of mid-to-high throughput assays. Using PIPETMAN technology, the robot transfers liquids with high accuracy and precision, helping to eliminate user-to-user variability.
"After PIPETMAX was introduced, the efficiency of reagent dispensing was much higher than using manual pipettes. We also use PIPETMAN® M and PLATEMASTER, but we will introduce additional PIPETMAX instruments to optimize our work. We feel that PIPETMAX is very useful for the automation of the preparation process since it has great operability and helps eliminate human error," says Kazuyama.
Initially, the center processed between 2,000-3,000 samples per day, but with the PIPETMAX automated pipetting system and additional personnel, they now process up to 20,000 samples daily.
PIPETMAN® DIAMOND Tips
The center also uses PIPETMAN DIAMOND filter tips for both their automated and manual pipetting steps. PIPETMAN DIAMOND filter tips help prevent aerosol-born contamination.
"We can use PIPETMAN DIAMOND tips for both our manual pipettes and our automation equipment. We have been able to continue our test process without being interrupted because we can manage our stock of tips by narrowing down the tip models to two kinds. Gilson's cooperation is indispensable for continuing our business." says Kazuyama.
Next Steps for SB Coronavirus Inspection Center Corporation
When asked about the next steps for the SB Coronavirus Inspection Center Corporation in the fight against COVID-19, Kazuyama responded, "Currently, we are contributing to creating an environment where people can live safely by providing a low-cost, high-volume saliva-based PCR testing service. We hope that we can terminate this business as soon as possible after a sufficient number of people in Japan are vaccinated and the infection of Coronavirus has completely ended."
Want to Learn More?
We want to enable you to be as efficient, and safe as possible using the most reliable sample prep technology on the market. Contact us to learn more about our capabilities and how we can help you scale up your COVID-19 testing workflows.
CONTACT US